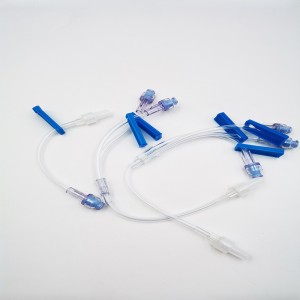
Ngwa ngwa mkpofu ọgwụ IV Set Extension Tube Flow Regulator Infusin Set



Onye na-ahụ maka ịgbasa mmiri nke ọma nwere tube ndọtị bụ ngwaọrụ ahụike eji ebugara onye ọrịa ikuku oxygen na-achịkwa.A na-ejikarị ya na ntọala dịka ụlọ ọgwụ, ụlọ ọgwụ, na nlekọta ụlọ.

Kpọmkwem ihe na-achịkwa usoro ịgbasa mmiri nwere tube ndọtị
1) Ihe
Tube: PVC na-agbanwe, NON-DEHP 4.0X2.7mm (ị nwere ike ịhọrọ PVC nkịtị ma ọ bụ na DEHP)
Onye na-achịkwa usoro mmiri: ABS, GASKET SILICON
Njikọ nwanyị/Nwoke: PC ihe nwere Lipid eguzogide
Isi: PE
Ebe Y na-enweghị agịga: PC, silicon, lipid eguzogide
Nkwakọ ngwaahịa: Soft Blister ma ọ bụ PE akpa
2) Akụkụ
Ọnụnọ na-agba ọsọ mgbe niile: 5 ruo 250 ml / awa na ndidi 10% na ọtụtụ.
PVC na-agbanwe, dị mfe iji, enweghị kink.
Nchekwa, enweghị agịga merụrụ ahụ maka mgbakwunye mmiri ọgwụ ahụike, Enweghị DEHP maka onye ọrịa. Kamma maka nọọsụ na onye ọrịa
Ihe niile na-akwado lipid eguzogide ọgwụ na mmanya na-eguzogide ọgwụ. Enweghị nchekasị maka ịwụpụ n'oge eji ma ọ bụ injection lipid.
Njikọ niile na-ezute 6:100 mba ụwa. Enweghị nchekasị maka nsogbu dakọtara.
CE
ISO 13485
TS EN ISO 13485: 2016 / AC: 2016 Sistemụ njikwa ogo akụrụngwa ọgwụ maka iwu chọrọ
TS EN ISO 14971 Ngwa ahụike - Ngwa njikwa ihe egwu na ngwaọrụ ahụike
TS EN ISO 11135: 2014 Ngwa ahụike ọgwụ mgbochi nke ethylene oxide nkwenye na njikwa izugbe
ISO 6009: 2016 agịga injection na-adịghị mma nke a na-atụfu ya Chọpụta koodu agba
TS EN ISO 7864: 2016 agịga injection na-enweghị atụ
TS EN ISO 9626: 2016 ọkpọkọ agịga igwe anaghị agba nchara maka imepụta ngwaọrụ ahụike

SHANGHAI TEAMSTAND CORPORATION bụ onye na-eweta ngwaahịa na ngwọta ahụike.
N'ihe karịrị afọ 10 nke ahụmịhe inye ahụike, anyị na-enye nhọrọ ngwaahịa dị ukwuu, ọnụahịa asọmpi, ọrụ OEM pụrụ iche, yana nnyefe oge a pụrụ ịdabere na ya.Anyị abụrụla onye na-eweta ngalaba ahụike gọọmentị Australia (AGDH) na ngalaba ahụike ọha na California (CDPH).Na China, anyị nọ n'ọkwa n'etiti ndị na-eweta Infusion, Injection, Vascular Access, Rehabilitation Equipment, Hemodialysis, Biopsy Needle na Paracentesis ngwaahịa.
Site na 2023, anyị enyefela ndị ahịa ngwaahịa na mba 120+ nke ọma, gụnyere USA, EU, Middle East, na Southeast Asia.Omume anyị kwa ụbọchị na-egosipụta nraranye anyị na nnabata anyị maka mkpa ndị ahịa, na-eme ka anyị bụrụ onye mmekọ azụmahịa tụkwasịrị obi yana jikọtara ọnụ.

Anyị enwetala aha ọma n'etiti ndị ahịa a niile maka ezigbo ọrụ na ọnụahịa asọmpi.

A1: Anyị nwere ahụmịhe afọ 10 na mpaghara a, ụlọ ọrụ anyị nwere otu ndị ọkachamara na ahịrị mmepụta ọkachamara.
A2.Ngwaahịa anyị nwere oke mma na ọnụahịa asọmpi.
A3.Usually bụ 10000pcs;anyị ga-achọ ka gị na gị na-arụkọ ọrụ, enweghị nchegbu banyere MOQ, dị nnọọ zitere anyị nke gị ihe ị chọrọ ịtụ.
A4.Ee, LOGO customization nabatara.
A5: Dị ka ọ na-adịkarị, anyị na-edobe ọtụtụ ngwaahịa na ngwaahịa, anyị nwere ike ibupu ihe nlele na 5-10workdays.
A6: Anyị na-ebufe site FEDEX.UPS, DHL, EMS ma ọ bụ oké osimiri.