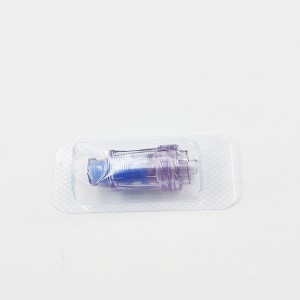
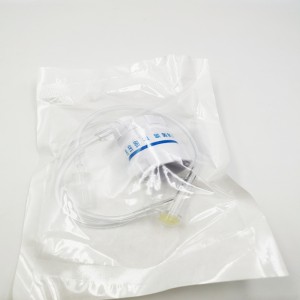
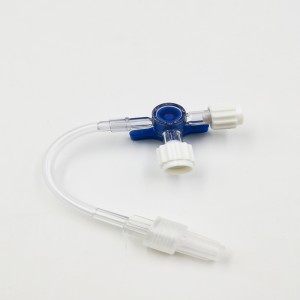
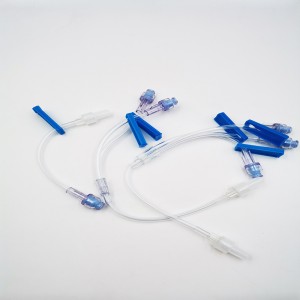
Ngwaahịa ọgwụ a na-atụfu nke IV Set IV Extension Tube Flow Regulator Infusin Set



Ihe na-achịkwa usoro mmiri Precise nke nwere tube ndọtị bụ ngwaọrụ ahụike eji ebuga oxygen n'ahụ onye ọrịa. A na-ejikarị ya eme ihe n'ebe dịka ụlọ ọgwụ, ụlọ ọgwụ, na nlekọta ụlọ.

Ihe njikwa mmiri kpọmkwem nwere ọkpọkọ ndọtị
1) Ihe eji eme ihe
Tubu: PVC na-agbanwe agbanwe, NỌGHỊ DEHP 4.0X2.7mm (ị nwere ike ịhọrọ PVC nkịtị ma ọ bụ DEHP)
Ihe na-achịkwa mmiri: ABS, GASKET SILICON
Njikọ Nwanyị/Nwoke: Ihe PC nwere lipid na-eguzogide ọgwụ
Mkpu:PE
Ebe a na-enweghị agịga Y: PC, silicon, lipid resistant
Ihe eji echekwa ihe: Akpa Soft Blister ma ọ bụ PE
2) Atụmatụ
Ọnụego mmiri na-aga n'ihu: 5 ruo 250 ml / awa yana ndidi 10% na oke.
PVC na-agbanwe agbanwe, dị mfe iji, enweghị kink.
Nchekwa, enweghị mmerụ ahụ agịga maka mgbakwunye mmiri ọgwụ, Enweghị DEHP maka onye ọrịa. Ọ ka mma maka nọọsụ na onye ọrịa
Ihe niile dị na ya na-akwado iguzogide lipid na iguzogide mmanya. Echegbula onwe gị maka mmiri mmiri mgbe ejiri ya ma ọ bụ ntụtụ lipid.
Njikọ niile na-ezute mba ụwa 6:100. Echegbula maka nsogbu dakọtara.
CE
ISO13485
EN ISO 13485: 2016/AC: 2016 Sistemụ njikwa mma akụrụngwa ahụike maka ihe ndị achọrọ maka iwu
EN ISO 14971: 2012 Ngwaọrụ Ahụike - Itinye njikwa ihe egwu n'ọrụ na ngwaọrụ ahụike
ISO 11135: 2014 Ngwa ọgwụ Mgbochi nke ethylene oxide Nkwenye na njikwa izugbe
ISO 6009: 2016 Agịga ntụtụ a na-atụfu ehichapụrụ ehichapụ Chọpụta koodu agba
ISO 7864: 2016 agịga a na-atụfu nke a na-atụfu
ISO 9626: 2016: Tubọn agịga anaghị agba nchara maka imepụta ngwaọrụ ahụike

SHANGHAI TEAMSTAND CORPORATION bụ ụlọ ọrụ na-enye ngwaahịa na ngwọta ahụike kachasị elu.
Site na ahụmịhe ọrụ nlekọta ahụike karịrị afọ iri, anyị na-enye ọtụtụ ngwaahịa, ọnụ ahịa asọmpi, ọrụ OEM pụrụ iche, yana nnyefe a pụrụ ịdabere na ya n'oge. Anyị abụrụla ndị na-ebubata ngwaahịa nke Ngalaba Ahụike nke Australia (AGDH) na Ngalaba Ahụike Ọha na Eze nke California (CDPH). Na China, anyị na-esonye n'ime ndị na-enye ngwaahịa Infusion, Injection, Vascular Access, Rehabilitation Equipment, Hemodialysis, Biopsy Needle na Paracentesis.
Ka ọ na-erule afọ 2023, anyị ebugala ngwaahịa nye ndị ahịa na mba karịrị 120, gụnyere USA, EU, Middle East, na Southeast Asia. Ihe anyị na-eme kwa ụbọchị na-egosi nraranye anyị na nzaghachi anyị na mkpa ndị ahịa, na-eme ka anyị bụrụ onye mmekọ azụmaahịa a pụrụ ịtụkwasị obi na nke jikọtara ọnụ nke anyị họọrọ.

Anyị enwetala aha ọma n'etiti ndị ahịa a niile maka ọrụ dị mma na ọnụ ahịa asọmpi.

A1: Anyị nwere ahụmịhe afọ 10 n'ọhịa a, ụlọ ọrụ anyị nwere ndị otu ọkachamara na ahịrị mmepụta ọkachamara.
A2. Ngwaahịa anyị nwere oke mma na ọnụ ahịa asọmpi.
A3. Ọ na-abụkarị 10000pcs; anyị ga-achọ iso gị rụkọọ ọrụ, echegbula onwe gị maka MOQ, zitere anyị ihe ịchọrọ ịtụ.
A4. Ee, a na-anabata nhazi LOGO.
A5: Dịka ọ na-adịkarị, anyị na-edebe ọtụtụ ngwaahịa n'ụlọ ahịa, anyị nwere ike izipu ihe nlele n'ime ụbọchị ọrụ 5-10.
A6: Anyị na-ebufe site na FEDEX.UPS,DHL,EMS ma ọ bụ Oké Osimiri.