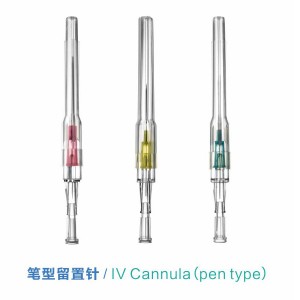
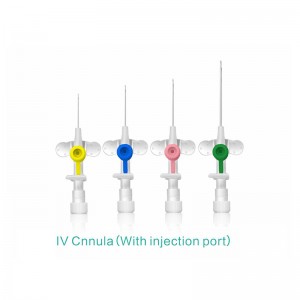
Sistemụ Katheta A Na-atụfu Emechiri Emechi nke Ahụike nwere njikọ



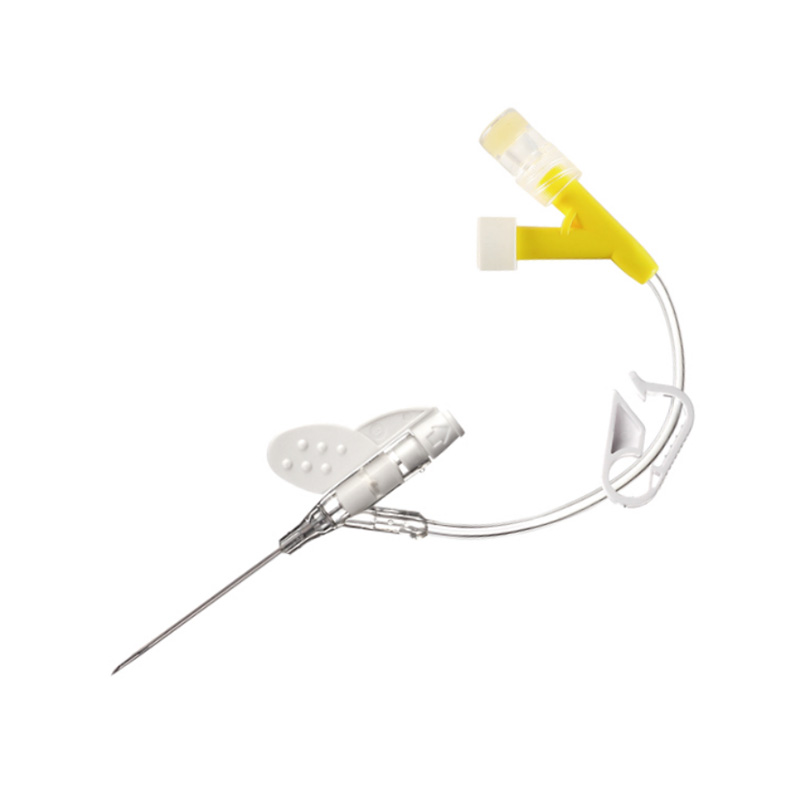
E mepụtara "Sistemụ Katheter nke Anọ Emechiri Emechi" iji belata ohere nke ibute ọrịa na ikpughe ọbara n'oge ịbanye n'ime akwara. O nwere sistemu mechiri emechi nke nwere valvụ arụnyere n'ime ya nke na-ebelata ntapụ ọbara ma gbochie mmetọ. A na-ejikarị sistemu a eme ihe n'ụlọ ọgwụ maka inye mmiri mmiri, ọgwụ, na ngwaahịa ọbara, ebe ọ na-eme ka nchekwa onye ọrịa ka mma. Ọ na-ebelatakwa mkpa ọ dị ịgbanwe katheter ugboro ugboro, na-eme ka ọ bụrụ nhọrọ a na-ahọrọ maka ọgwụgwọ inje ogologo oge na nlekọta dị oke mkpa.
Nkọwa Ngwaahịa:
Nha nkọwapụta: 16G, 18G, 20G, 22G, 24G na 26G
atụmatụ
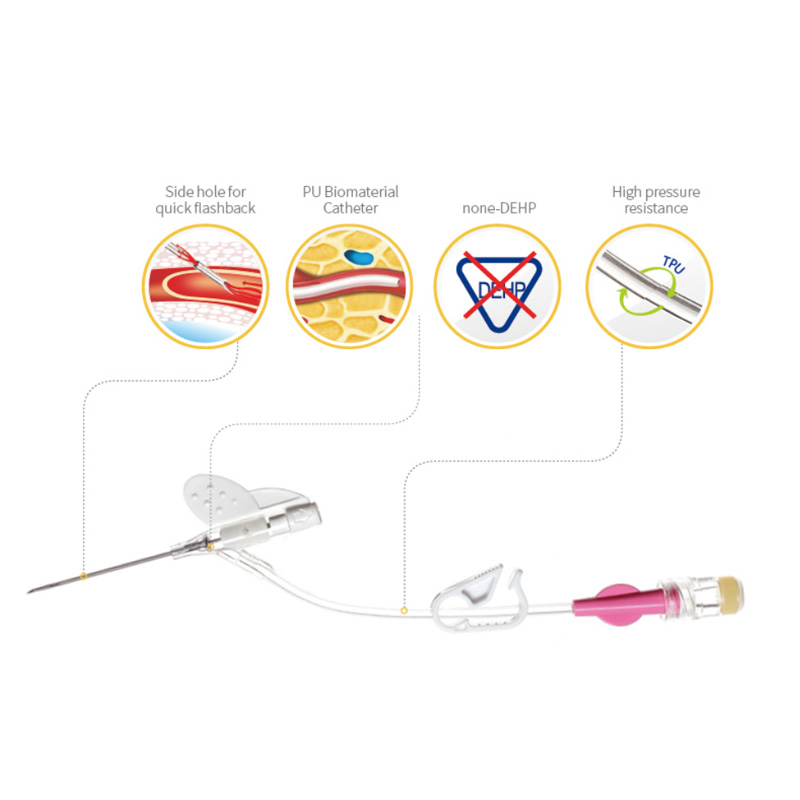
Agịga dị nkọ nwere usoro Universal Bevel nke na-enye ohere ịhọrọ akụkụ ntinye sara mbara, na-enye ohere ịgbanwe ihe.
"Flashback" "windo" maka ịhụ ọbara ngwa ngwa.
Kateter biomaterial PU maka ogologo oge obibi.
MDR 2017/745
Ụlọ Ọrụ FDA nke USA 510K
EN ISO 13485: 2016/AC: 2016 Sistemụ njikwa mma akụrụngwa ahụike maka ihe ndị achọrọ maka iwu
EN ISO 14971: 2012 Ngwaọrụ Ahụike - Itinye njikwa ihe egwu n'ọrụ na ngwaọrụ ahụike
ISO 11135: 2014 Ngwa ọgwụ Mgbochi nke ethylene oxide Nkwenye na njikwa izugbe
ISO 6009: 2016 Agịga ntụtụ a na-atụfu ehichapụrụ ehichapụ Chọpụta koodu agba
ISO 7864: 2016 agịga a na-atụfu nke a na-atụfu
ISO 9626: 2016: Tubọn agịga anaghị agba nchara maka imepụta ngwaọrụ ahụike

SHANGHAI TEAMSTAND CORPORATION bụ ụlọ ọrụ na-enye ngwaahịa na ngwọta ahụike kachasị elu.
Site na ahụmịhe ọrụ nlekọta ahụike karịrị afọ iri, anyị na-enye ọtụtụ ngwaahịa, ọnụ ahịa asọmpi, ọrụ OEM pụrụ iche, yana nnyefe a pụrụ ịdabere na ya n'oge. Anyị abụrụla ndị na-ebubata ngwaahịa nke Ngalaba Ahụike nke Australia (AGDH) na Ngalaba Ahụike Ọha na Eze nke California (CDPH). Na China, anyị na-esonye n'ime ndị na-enye ngwaahịa Infusion, Injection, Vascular Access, Rehabilitation Equipment, Hemodialysis, Biopsy Needle na Paracentesis.
Ka ọ na-erule afọ 2023, anyị ebugala ngwaahịa nye ndị ahịa na mba karịrị 120, gụnyere USA, EU, Middle East, na Southeast Asia. Ihe anyị na-eme kwa ụbọchị na-egosi nraranye anyị na nzaghachi anyị na mkpa ndị ahịa, na-eme ka anyị bụrụ onye mmekọ azụmaahịa a pụrụ ịtụkwasị obi na nke jikọtara ọnụ nke anyị họọrọ.

Anyị enwetala aha ọma n'etiti ndị ahịa a niile maka ọrụ dị mma na ọnụ ahịa asọmpi.

A1: Anyị nwere ahụmịhe afọ 10 n'ọhịa a, ụlọ ọrụ anyị nwere ndị otu ọkachamara na ahịrị mmepụta ọkachamara.
A2. Ngwaahịa anyị nwere oke mma na ọnụ ahịa asọmpi.
A3. Ọ na-abụkarị 10000pcs; anyị ga-achọ iso gị rụkọọ ọrụ, echegbula onwe gị maka MOQ, zitere anyị ihe ịchọrọ ịtụ.
A4. Ee, a na-anabata nhazi LOGO.
A5: Dịka ọ na-adịkarị, anyị na-edebe ọtụtụ ngwaahịa n'ụlọ ahịa, anyị nwere ike izipu ihe nlele n'ime ụbọchị ọrụ 5-10.
A6: Anyị na-ebufe site na FEDEX.UPS,DHL,EMS ma ọ bụ Oké Osimiri.